Rapid ‘Ōhi‘a Death encompasses two newly-described diseases, Ceratocystis wilt of ‘ōhi‘a (caused by the pathogenic fungus Ceratocystis lukuohia) and Ceratocystis canker of ‘ōhi‘a (caused by the pathogenic fungus Ceratocystis huliohia). Both fungal species are newly named and were formerly grouped into the species Ceratocystis fimbriata sensu lato.
(‘Ōhi‘a trees are also under threat from ʻōhiʻa or Austropuccinia rust, which has been on all the major Hawaiian islands since 2005; please see separate Gallery write-up on ʻōhiʻa or Puccinia rust here.)
Scientists became aware of ROD in 2010, when they detected rapid mortality affecting Hawaii’s most widespread and common tree, ʻōhiʻa lehua (Metrosideros polymorpha) on Hawai‘i Island. The Big Island has the most extensive ʻōhiʻa forests in the state (~250,00 ha). As of 2023, significant mortality has occurred on over 72,000 ha, or more than 1/3 of the vulnerable forest. While mortality has been detected across the entire island, it remains patchy. Plots in affected forest stands show from 3% to 50% mortality, and in the worst cases, over 90% of the ʻōhiʻa are dead. Forests in drier environments or at higher elevations seem to be less susceptible to the disease (Friday in West Side symposium; Luiz et al. 2023). In 2018 and 2019, scientists detected the disease on Kaua’i and later on Maui and O’ahu.
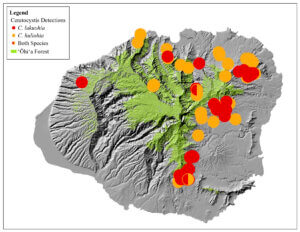
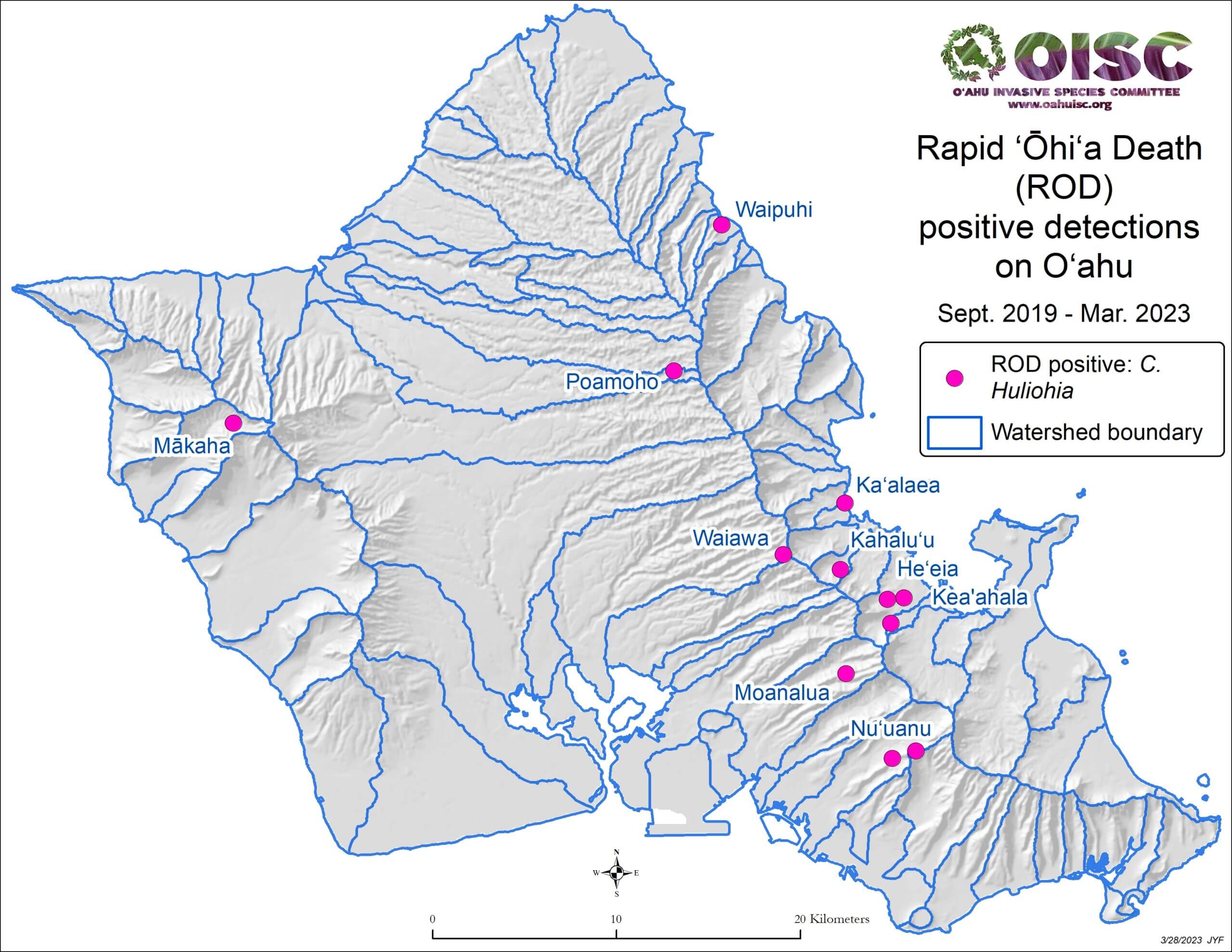
The cause was initially identified by the USDA Agriculture Research Service (ARS) as a new strain of Ceratocystis fimbriata, a vascular wilt fungus (Keith et al. 2015). Further genetic and morphological analysis revealed that the disease is caused by two previously unknown fungal species in the same genus: Ceratocystis lukuohia (which means “destroyer of ‘ōhi‘a” in the Hawaiian language) and C. huliohia (which means “overturns ‘ōhi‘a” in the Hawaiian language). C. lukuohia causes a true vascular wilt and is more virulent; C. huliohia causes cankers which can gradually girdle and kill trees. These fungi did not evolve from C. fimbriata already on islands (Barnes et al. 2018). C lukuohia belongs to a Latin America clade, C. huliohia to an Asian-Australian clade. These origins suggest they were introduced separately. Both are now spread across Hawai’i and Kaua’i. Only the less virulent C. huliohia has been detected on O’ahu, and Maui (with only a single detection), and there have been no detections on Moloka’i or Lana’i (Luiz et al. 2023) [see maps].
Upon discovery of the disease, the Hawai ‘i Department of Agriculture (HDOA) quickly adopted an emergency quarantine prohibiting movement from the Hawai’i Island of ʻōhiʻa lehua flowers, leaves, twigs, and wood (all plants and parts), mulch, and greenwaste of any species in the same genus as ʻōhiʻa (Metrosideros), except under terms of a permit issued by the HDOA. Soil was added to the quarantine in January 2016. https://hdoa.hawaii.gov/blog/main/ohiaquarantine/ and https://hdoa.hawaii.gov/blog/main/nr-rodsoil/
‘Ōhi‘a trees overwhelmingly dominate approximately 80% of Hawaii’s remaining native forest in both wet and dry habitats. Since arrival of the ancestral tree more than 3 million years ago on the island of Kaua’i, the tree has colonized the other main Hawaiian Islands as they emerged. Adaptive radiation led to multiple Metrosideros species occupying different ecological niches across the islands. Five species are now recognized. One, M. polymorpha, is found throughout the state. There are eight recognized varieties that have adapted to selective pressures characteristic of the varied locations.
Scientists hypothesize that ʻōhiʻa is undergoing incipient speciation. Luiz et al (2023) list the other Metrosideros taxa and the islands on which they occur.
Little is known about the susceptibility of other Hawaiian Metrosideros taxa to C. lukuohia and C. huliohia. To date, the diseases have been reported on only M. polymorpha. Scientists have begun screening seedlings from endemic Kaua’i and O’ahu Metrosideros species (Luiz et al. 2023).
Loss of ʻōhiʻa lehua could result in significant changes to the structure, composition, and potentially, the function, of forests on a landscape level. ‘Ōhi‘a forests are home to the Islands’ one native terrestrial mammal (Hawaiian hoary bat) and 30 species of forest birds – especially the unique honeycreeper endemic subfamily. Eighteen of 19 extant Hawaiian honeycreepers in the main Hawaiian islands, including 12 of 13 species listed as endangered by the U.S. Fish and Wildlife Service, depend on ‘ōhi‘a for critical habitat (Loope and LaRosa, 2008). Also at risk are more than 500 endemic arthropod species, many of which are also endangered or threatened (Luiz et al. 2023). The increased light penetrating interior forests following canopy dieback facilitates invasion by light-loving non-native plant species, of which HI has dozens. Loss of ʻōhiʻa also damage habitat for one-third to one-half of Hawaii’s approximately 300 endangered plant species (Loope and LaRosa, 2008) through encouraging non-native competitors and changing understory environmental conditions. More than half of Hawaii’s 553,000 ha of forest land contain non-native tree species; about 39% of these forest lands are dominated by non-native tree species. Two-thirds of saplings (63%) and seedlings (66%) are non-native (Patter et al. 2023). These figures do not include invasive plants of other growth forms. There is perhaps no other species in the United States that supports more endangered taxa or that plays such a geographical dominant ecological keystone role (Luiz et al. 2023).
Hawai’i depends on groundwater for freshwater for residential and agriculture. High elevation ‘ohi’a forests protect watersheds across the state, and, because of their lower water usage compared to fast-growing non-native species, allow for greater recharge of groundwater (Luiz et al. 2023).
For many Native Hawaiians, ‘ōhi‘a is a physical manifestation of multiple Hawaiian deities and the subject of many Hawaiian proverbs, the subject of an enormous number of chants and stories, and foundational to the scared practice of many hula. The wood is used in constructing temples and traditional Hawaiian houses, as tool and weapon handles, and carvings of Hawaiian gods. It is also sought as firewood. Flowers, shoots, and aerial roots are used medicinally to treat many ailments. Flowers and shoots are also used for making lei and intrinsic beauty. The biocultural link between ‘ohi‘a and the people of Hawai`i is a defining feature of Hawaii’s socio-ecological landscapes (Loope and LaRosa, 2008; Luiz et al. 2023).
‘Ōhi‘a can become infected only if the fungus can enter through a wound. Such wounds can be caused by damage resulting from wind, animals (especially domestic or feral ungulates which strip bark from the trees), construction equipment, and pruning. ‘Ōhi‘a mortality rates are higher in stands where ungulates are present (Perroy et al.). ‘Ōhi‘a trees do not seem to take up the pathogen through their roots (Yelenik et al. 2020).
Dispersal pathways are not completely understood. Ambrosia beetles attack infected trees, and their frass can contain viable spores of C. lukuohia (Roy et al. 2018, 2020). While the initial focus was on windblown frass, new evidence suggests that ambrosia beetles can directly vector fungal propagules between trees (Roy et al. 2023). It is not yet understood how frequently this occurs, or how important this pathway is in disease transmission (Luiz et al. 2023). Human-assisted dispersal is suspected in the form of contaminated tools and movement of infected firewood, fence posts, and other plant parts, and soil – including on the tires and undercarriage of vehicles. Human activities also create infection courts through wounding the tree – either by human actions directly or by livestock and other, usually feral, ungulates (see above).
Considerable effort has been put into mapping the spread of the disease. Because of the large areas that must be surveyed and the difficult terrain, scientists are seeking to improve detection from aerial platforms, including fixed-wing planes (Asner et al. 2018) helicopters, and – especially drones. Drone cameras can pinpoint suspect trees’ location so scientists doing the essential ground-based sampling can find them most easily. Drones also can track individual trees’ decline over time, facilitating analysis of links between the diseases and land use, environmental conditions, and other factors. The USDA Forest Service Region 5 and state agencies have supported attempts to develop spectral-chemical signatures that can distinguish individual tree crowns’ symptoms from the air – symptoms of active infection (brown/desiccated crowns) as well as past infection (leafless tree crowns) (Perroy et al. 2020). Success would enable both landscape-scale, spatially explicit maps of probable ROD for early detection and tracking spread, as well as identification of putative survivor trees to be incorporated into tests of natural resistance and possible future breeding programs (Luiz et al. 2023). Current work is additionally investigating whether satellite images can be used to track ROD. Repeated measurements over time in affected forests show initial high rates of mortality decreasing over time but not approaching zero.
Currently, there is no effective treatment to either protect ʻōhiʻa trees or to cure them (College of Tropical Agriculture 2015, see www.RapidOhiaDeath.org). Tests using propiconazole injected into trees as a prophylactic showed mixed results, and drilling holes into trees to use as injection ports may do more harm than good.
The fact that some ‘ōhi‘a survive in forests on the Big Island in the presence of ROD indicates that some trees might possess natural resistance. These asymptomatic potentially “survivor” trees are prime candidates for screening efforts. Scientists are collecting germplasm from these lightly impacted stands near high-mortality stands. In addition to being comprised of potentially disease-resistant trees which should be protected from other threats, such stands represent sites for further study of disease infection and progression (Luiz et al. 2023).
Because of the ecological importance of ‘ōhi‘a and the rapid spread of this lethal disease, research into possible resistance to the disease began almost immediately in 2016. In a preliminary assessment by the University of Hawai‘i at Hilo and the USDA Agriculture Research Service, five of 124 seedlings representing four varieties of M. polymorpha. survived three years after exposure to the disease. These seedlings are used to produce rooted cuttings and seeds for further evaluation of these genotypes. These indications are promising, although more comprehensive screening of trees from throughout species’ range is needed to provide an accurate baseline on the frequency, level, and the distribution of genetic resistance to both pathogens.
Encouraged by these developments, and recognizing the scope of additional work needed, stakeholders created a collaborative partnership in 2018 that includes state, federal, and non-profit agencies and entities: ‘Ōhi‘a Disease Resistance Program (‘ODRP) (Luiz et al. 2023) (https://www.akakaforests.org/programs/ohia-disease-resistance-program). The partnership seeks to provide baseline information on genetic resistance present in all Hawaiian taxa in the genus Metrosideros. It aims further to develop sources of ROD-resistant germplasm for various restoration purposes including cultural plantings, landscaping, and ecological restoration. ‘ODRP is pursuing these goals through (Luiz et al. 2023) 1) evaluating and operationalizing methods for inoculation-based screening and greenhouse-based production of test plants, and 2) short-term greenhouse screenings of seedlings and rooted cuttings sampled from native Metrosideros throughout Hawai’i. To date, inoculation techniques have been improved and hundreds of seedlings and rooted cuttings have been screened, with many showing signs of resistance to the disease.
ODRP will then expand its program to:
- establish field trials, with the help of local communities, to validate the short-term greenhouse assays and monitor durability and stability of resistance
- research to understand environmental and genetic (vascular architecture, wound response) drivers of susceptibility and resistance
- develop remote sensing and molecular methods to rapidly detect ROD-resistant individuals
- if necessary, conduct breeding to increase the efficacy of resistance and improve durability of ROD resistance
- support ongoing Metrosideros conservation work on important genotypes.
To be successful, ‘ODRP must surmount several challenges (Luiz et al. 2022):
- increase capacity to screen seedlings from several hundred plants per year to several thousand
- optimize artificial inoculation methodologies
- determine the effects of temperature and season on infection rates and disease progression, so that test results are not skewed by such factors
- find ways to speed up seedlings’ attaining sufficient size for testing
- develop improved ways to clone propagules
- improve efficiency of detecting putative survivor trees in the forest (see above)
- establish sites for field testing of putatively resistant trees (essential for examining the durability of resistance) and establishment of seed orchards; this includes sites across a wide range of climatic and edaphic conditions, preferably on the several islands so scientists can work with locally appropriate genetic varieties without risking transport of the pathogen to uninfected areas and in compliance with quarantine
- establish systems for seed collection from the wide variety of subspecies/varieties (hope to rely on collaborators, including school groups ‘ohi’a is prolific seeder, so that is not a problem)
- if breeding to enhance resistance is appropriate, it will be useful to develop high-throughput phenotyping of the seed orchard plantings
Scientists hope to be able to produce M. polymorpha that are resistant to both C. lukuohia and C. huliohia. It is possible that resistance to one pathogen does not confer resistance to the other. If so, scientists will attempt to breed progeny that have resistance to both (Luiz et al. 2023).
Once these vitally important activities are under way, (Luiz et al. 2023) scientists can turn to research aimed at understanding the genetic basis of host defense mechanisms. As noted above, one group of questions concerns whether ROD is similar to DED in that host anatomical factors (i.e., width of xylem vessels) plays a role. Vessel diameters are smaller in ‘ohi’a at higher elevations. Might this factor, coupled with cooler temperatures, explain the less aggressive expansion of ROD seen in high elevation sites? Further research is planned to investigate the potential associations between climate, ‘ohi’a xylem architecture, and susceptibility to C. lukuohia.
Developing ROD-resistant ʻōhiʻa is only one part of the overall program to save Hawaii’s ‘ohi’a forests. Protecting healthy forests is cheaper and more effective than trying to restore forests that have already been hit with the disease. Luiz et al. (2023) reiterate the importance of maintaining/complying with quarantines on movement of infected material, increased education of forest users on the importance bio-sanitation, and countering activities that injure the trees. As noted above, areas from which feral ungulates have been expelled and fenced have much lower levels of disease. Fencing that protects these forests from feral animals can drastically reduce the amount of disease and can be applied on the scale of thousands of acres. Another issue is that there is almost no natural ʻōhiʻa regeneration in lower elevation forests, probably because of competition with invasive plants (see below) and the presence of diseases such as Austropuccinia psidii. Control of invasive plants and replanting with disease-resistant ʻōhiʻa and other native trees could restore limited areas of ecologically important forests and smaller forests managed by private landowners (Luiz et al. 2023).
A new assessment of the extent of invasive plant species in forests on the Hawaiian Islands (Potter et al. 2023) reached “sobering” results that portend “a more dire future for Hawaii`s native forests.” The authors analyzed plant presence data from 238 plots established under the USDA Forest Service’ Forest and Inventory Analysis (FIA) program https://www.fia.fs.usda.gov/. The findings are incomplete because a) landowners have refused access to islands (Moloka‘i, Kaho’olawe, and Ni‘ihau); and most xerophytic (dry) forests had already been converted to grasslands before the FIA surveys began. In the wetter forests surveyed:
- 56% of Hawaii’s 553,000 ha of forest land contained non-native tree species; about 39% of these forest lands are dominated by non-native tree species.
- About two-thirds of saplings (63%) and seedlings (66%) in forests across the Islands are non-native. Potter et al. (2023) expect that plant succession will result in transformation of these forests’ canopies from native tree species to non-native species.
- 75% of forests in lower-elevation areas of all islands and 31% of higher-elevation forests are already dominated by non-native tree species.
- Widescale replacement of native trees by non-native species is likely. Several factors favor these changes: 1) the impact of Rapid ‘Ōhi‘a Death; 2) invasions by forbs and grasses; 3) soil damage and other disturbances caused by invasive ungulates; and 4) climate change. If succession conforms to these trends, non-native tree species could eventually constitute 75% or more of the forest tree stems and basal area on all islands and across forest types and elevations.
Sources
Asner, G.P, R.E. Martin, L.M. Keith, W.P. Heller, M.A. Hughes, N.R. Vaughn, R.F. Hughes, and C. Balzotti. 2018. A spectral mapping signature for the Rapid Ohia Death (ROD) pathogen in Hawaiian forests. Remote Sensing 10:404 – 428. doi: 10.3390/rs10030404
Atkinson, C.T. and K. Roy. 2023. Environmental monitoring for invasive fungal pathogens of ‘Ōhi‘a (Metrosideros polymorpha) on the Island of Hawai`i. Biological Invasions (2023) 25:399–410
https://doi.org/10.1007/s10530-022-02922-3
Barnes, I., A. Fourie, M.J. Wingfield, T.C. Harrington, D.L. McNew, L.S. Sugiyama, B.C. Luiz, W.P. Heller, and L.M. Keith. 2018. New Ceratocystis species associated with rapid death of Metrosideros polymorpha in Hawai‘i. Persoonia 40: 154-181. https://doi.org/10.3767/persoonia.2018.40.07
Hughes, M. 2018 Rapid ʻŌhiʻa Death Symposium -West Hawaiʻi March 3rd 2018, https://vimeo.com/258674532 Accessed April 4, 2018 (see also full video archive at https://vimeo.com/user10051674)
Friday, J. B., L. Keith, and F. Hughes. 2015. Rapid ‘Ōhi‘a Death (Ceratocystis Wilt of ‘Ōhi‘a). PD-107, College of Tropical Agriculture and Human Resources, University of Hawai‘i, Honolulu, HI. URL: https://www.ctahr.hawaii.edu/oc/freepubs/pdf/PD-107.pdf Accessed April 3, 2018.
Friday, J.B. 2018. Rapid ʻŌhiʻa Death Symposium -West Hawaiʻi (“West Side Symposium”) March 3rd 2018, https://vimeo.com/258704469 Accessed April 4, 2018 (see also full video archive at https://vimeo.com/user10051674)
Hughes, M. 2018 Rapid ʻŌhiʻa Death Symposium -West Hawaiʻi March 3rd 2018, https://vimeo.com/258674532 Accessed April 4, 2018 (see also full video archive at https://vimeo.com/user10051674)
Keith, L.M., R. F. Hughes, L. S. Sugiyama, W. P. Heller, B. C. Bushe, and J. B. Friday. 2015. First report of Ceratocystis wilt on ʻōhiʻa. Plant Disease. http://dx.doi.org/10.1094/PDIS-12-14-1293-PDN
Loope, L. and A.M. LaRosa. 2008. ‘Ohi’a Rust (Eucalyptus Rust) (Puccinia psidii Winter) Risk Assessment for Hawai`i
Luiz, B.C. 2017. Understanding Ceratocystis species A: Growth, morphology, and host resistance. MS thesis, University of Hawai‘i at Hilo.
Luiz, B.C., C.P. Giardina, L.M. Keith, D.F. Jacobs, R.A. Sniezko, M.A. Hughes, J.B. Friday, P. Cannon, R. Hauff, K. Francisco, M.M. Chau, N. Dudley, A. Yeh, G. Asner, R.E. Martin, R. Perroy, B.J. Tucker, A. Evangelista, V. Fernandez, C. Martins-Keli’iho.omalu, K. Santos, R. Ohara. 2023. A framework for establishlishing a rapid ‘Ohi‘a death resistance program New Forests 54, 637–660. https://doi.org/10.1007/s11056-021-09896-5
Perroy, R.L., M. Hughes, L.M. Keith, E. Collier, T. Sullivan, and G. Low. 2020. Examining the utility of visible near-infrared and optical remote sensing for the early detection of Rapid ‘Ōhi‘a Death. Remote Sensing 12; doi:10.3390/rs12111846.
Perroy, R.L., T. Sullivan, D. Benitez, R.F. Hughes, L.M. Keith, E. Brill, K. Kissinger and D. Duda. 2021. Spatial patterns of ‘ōhi‘a mortality associated with Rapid ‘Ōhi‘a Death and ungulate presence. Forests, 12, 1035. https://doi.org/10.3390/f12081035.
Potter, K.M., Escanferla, M.E., Jetton, R.M., Man, G., Crane, B.S., Prioritizing the conservation needs of US tree spp: Evaluating vulnerability to forest insect and disease threats, Global Ecology and Conservation (2019), doi: https://doi.org/10.1016/
Potter, K.M., C. Giardina, R.F. Hughes, S. Cordell, O. Kuegler, A. Koch, E. Yuen. 2023. How invaded are Hawaiian forests? Non-native understory tree dominance signals potential canopy replacement. Landscape Ecology 38, 3903–3923 (2023). https://doi.org/10.1007/s10980-023-01662-6
Roy, K., C.P. Ewing, M.A. Hughes, L. Keith, and G.M. Bennett. 2018. Presence and viability of Ceratocystis lukuohia in ambrosia beetle frass from Rapid ʻŌhiʻa Death-affected Metrosideros polymorpha trees on Hawaiʻi Island. Forest Pathology 2018;e12476. https://doi.org/10.1111/efp.12476. Forest Pathology e12476. https://doi.org/10.1111/efp.12476
Roy, K., K.A. Jaeneke, and R.W. Peck. 2020. Ambrosia beetle (Coleoptera: Curculionidae) communities and frass production in ‘ōhi‘a (Myrtales: Myrtaceae) infected withh Ceratocystis (Microascales: Ceratocystidaceae) fungi responsible for Rapid ʻŌhiʻa Death. Environmental Entomology 2020 1-10, doi: 10-1093.
Roy, K., K. A. Jaenecke, E. J. Dunkle, D. Mikros, & R. W. Peck. (2023). Ambrosia beetles (Coleoptera: Curculionidae) can directly transmit the fungal pathogens responsible for Rapid ʻŌhiʻa Death. Forest Pathology, 00, e12812. https://doi.org/10.1111/efp.12812
Vaughn, N.R., G.P. Asner, P.G. Brodrick, R.E. Martin, J.W. Heckler, D.E. Knapp, and R.F. Hughes. 2018. An approach for high-resolution mapping of Hawaiian Metrosideros forest mortality using laser-guided imaging spectroscopy. Remote Sensing 10: 502-519. Doi: 10.3390/rs10040502
Yelenik, S.G., K.Roy, J. Stallman. 2020. Successful restoration of Metrosideros polymorpha (ʻōhiʻa) is possible in forest sites with active Rapid ʻŌhiʻa Death infections. Restoration Ecology, 28(5) 1257-1261. doi:10.1111/rec.13197