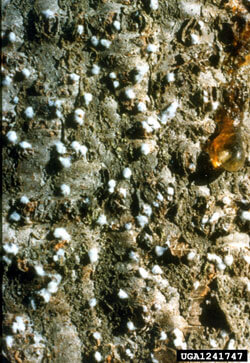
The balsam woolly adelgid (BWA) was introduced to New England before 1908 (Kotinsky, 1916) and the western states before 1928 (Annand, 1928), likely on nursery stock from Europe. North American true firs (Abies) vary in susceptibility and resistance. Feeding adelgids inject a toxic saliva which causes thickening of the tree host’s vascular tissues which interferes with the transport of water and nutrients, often leading to premature needle loss and crown dieback (Balch et al., 1964).
Spread of the adelgid is by transport of the mobile crawler stage by wind, animals, or people to new areas, or via movement of infested plants (Ragenovich and Mitchell, 2006; Kohler 2016)
Impacts in the Eastern US
The greatest mortality has been in the southern Appalachians where balsam woolly adelgid kills mature specimens of two narrowly endemic taxa, Fraser fir (A. fraseri) and A. balsamea phanerolepis.
Fraser fir is restricted to mountaintop environments in the southern Appalachians, where the species forms a unique forest type with red spruce (Picea rubens). Mortality was first noticed in 1957 (Johnson, 1980). A study on Mt. Guyot, Tennessee, in the Great Smoky Mountains National Park revealed that Fraser fir declined from 80% to 2.5% of living crown trees in the time period of 1967-1985 (Alsop and Laughlin, 1991). This demise resulted in a dramatic change in forest composition and dynamics on former Fraser fir sites. With the forest canopy removed, the understory changed from primarily blueberry (Vaccinium) and fir saplings to dense blackberry (Rubus), blueberry, and Viburnum. Increases occurred in the proportion of red spruce (P. rubens) and yellow birch (Betula alleghaniensis) in the forest canopy. The spruce-fir moss spider, Microhexura montivaga, and a narrowly endemic lichen, Gymnoderma lineare, that inhabit this unique habitat have been listed as a federally endangered species. Changes in avian species and populations also have been observed in other studies (cf. Rabenold et al., 1998).
Regeneration has been robust, but no one knows whether the trees will become vulnerable to the adelgid once they mature.
Control of the adelgids with insecticides in forested areas has not been successful or practical. Biological controls have been attempted (Schooley et al., 1984; Humble, 1994), but none have demonstrated satisfactory levels of success to date (Montgomery and Havill 2014). A planting of genotypes from different mountaintops was established in the Great Smoky Mountains National Park during the early 1990s to conserve the genetic resources of this fir species (S.E. Schlarbaum, pers. comm.).
According to the analysis carried out under the USDA Forest Service CAPTURE program (Conservation Assessment and Prioritization of Forest Trees Under Risk of Extirpation) (Potter et al. 2019), Fraser fir is one of six North American tree species that face severe pest threats and are highly sensitive to the relevant pest, but also have a high capacity to adapt. The project urges immediate conservation and the facilitation of resistance through breeding.
BWA is also killing balsam fir (Adelges balsamea) in the Northeastern U.S. and Canada. In 2017, USDA Forest Service aerial surveys detected mortality on 2,680 ha in the Northeastern U.S. (Potter and Conkling 2018).
The relict subspecies, A. balsamea phanerolepis, has a limited distribution in a few small, isolated stands in Tucker, Randolph, and Pocahontas counties of West Virginia. Balsam woolly adelgid was first detected in these stands in 1992, but dendrological analysis indicates it was probably present in the 1980s, possibly as early as the 1970s. The stands are small and easily overlooked so infestation may have been present earlier (Hessl, Liebhold, and Leef. 2022). By 1998, the adelgid was present and causing considerable tree mortality at all sites.
Hessl, Liebhold, and Leef (2022) call for efforts to search for biological control agents to protect relic A. balsamea phanerolepis populations; they cite the current focus on predaceous flies (Diptera: Chamaemyiidae) as possible biocontrol agents targetting the closely related hemlock woolly adelgid (Adelges tsugae).
Impacts in the Western US
In the West, the balsam woolly adelgid was reported in California in 1928, in Oregon around 1930 (Keen 1938), Washington in 1954 (Johnson and Wright 1957), and British Columbia in 1958 (Zilahi-Balogh et al., 2016). By 1983, BWA was detected in Idaho (Livingston et al., 2000) and it has spread to Montana, Utah (N.P. Havill, pers. comm.), and interior British Columbia (Zilahi-Balogh et al., 2016). In 2019, it was found in Juneau, Alaska. (https://www.fs.usda.gov/detailfull/r10/forest-grasslandhealth/?cid=FSEPRD686378)
Vulnerable hosts in the west are subalpine fir (Abies lasiocarpa), Pacific silver fir (A. amabilis), grand fir (A. grandis), and white fir (Abies concolor). An initial wave of mortality occurred in Cascades by the late 1950s -1960s (Kohler 2016). Mortality continues throughout both Oregon and Washington, most commonly in the Cascade and coastal mountain ranges, western valleys and lowlands, in the Blue Mountains of eastern Oregon and Washington, (Ragenovich and Mitchell, 2006) and where firs occur throughout southern Idaho and Utah (N.P. Havill, pers. comm.).
BWA is killing grand fir from low elevation areas of the Willamette Valley, Puget Sound trough, and along coastal streams, including those found in the Coast Range, coastal Siskiyou Mountains, and coastal lowland areas (Ragenovich and Mitchell, 2006) as well as in southwest Idaho (Lowrey 2015) and coastal British Columbia (Humble pers. comm.). On the Olympic Peninsula, silver fir near sea level has not experienced as extensive damage; it is not known why (Kohler 2016). Subalpine fir has been attacked across the peninsula (Hutton 2015). Still, approximately 50% of fir plots in Oregon still lacked symptoms of BWA nearly 80 years after first detection, not because of tree resistance, but because of the pattern of spread (Hutton 2015).
BWA is also removing subalpine fir as a pioneer species in important mountain environments such as alpine meadows, avalanche chutes, and lava beds. In certain situations, subalpine fir is the only tree species capable of colonizing these harsh environments. In other situations, successional pathways are altered, presumably for the long-term (Ragenovich and Mitchell, 2006).
Subalpine fir – although the most susceptible – remains at high altitude timberline locations (Ragenovich and Mitchell, 2006). Higher elevation forests have already been severely damaged by white pine blister rust, which has killed many of the whitebark pine (Pinus albicaulus). Link to WPBR writeup
According to Pederson et al. (no date), disappearance of high elevation subalpine fir stands may have the following adverse effects on the ecosystem:
- Hydrological effects, including reduced ground water retention, increased rate of snow melt, and altered stream flow characteristics.
- Increased fuel loads and thus greater fire danger caused by dead trees.
- Wildlife may be affected by changes in stand composition.
- Loss of the tree canopy along streams can impact water quality and fish populations downstream.
BWA causes the greatest damage on hosts growing at the low elevations for their species ranges, and on wet sites (Ragenovich and Mitchell, 2006). However, host trees are readily killed across dry forests of southern Idaho (Lowrey 2015). Surviving trees often have crown dieback, top-kill, reduced and deformed growth, diminished seed production and are more susceptible to secondary attacks by pests (e.g. bark beetles and spruce budworm). They also suffer reduced reproduction) and droughty conditions Lowrey 2015).
Aerial pest surveys have weaknesses. First, only new tree damage is documented each year, so the data do not track cumulative mortality or temporal patterns (Hutton 2015). Furthermore, only some areas are surveyed each year; mortality in unsurveyed areas is not reported – although it continues! In addition, individual observers’ mapping procedures might vary. Finally, other factors contribute to mortality of subalpine fir, including western bark beetle and possibly root disease (https://www.idl.idaho.gov/wp-content/uploads/sites/116/2020/04/2019-Idaho-Forest-Health-Highlights.pdf). Nevertheless, the surveys document extensive impacts.
The ten-year average of fir mortality between 2005 and 2015 was 109,000 acres in Oregon, 35,000 acres in Washington (Kohler 2016). The Washington average was lower in the decade 2010-2019: 30,000 acres per year, especially at high elevations of the Blue Mountains, Olympic Mountains, scattered areas along crest of Cascade Mountains and the mountains of northeast Washington (https://www.dnr.wa.gov/ForestHealth select “ highlights” along right sidebar)
Several attempts have been made to find biocontrol agents. Twenty-five predator species were introduced in the United States and Canada during the 1950s and 1960s. Eight species established. However, although abundant, neither introduced nor native predators are effective in controlling BWA or reducing damage. (Kohler 2016; Buhl)
Additional threat to Fraser firs
Scientists in Connecticut have detected a previously unknown species of water mold in the genus Phytophthora on trees being grown in a breeding experiment. (APS)
USFS scientists and managers developed a conservation priority-setting framework for forest tree species at risk from pest & pathogens and other threats. The Project CAPTURE (Conservation Assessment and Prioritization of Forest Trees Under Risk of Extirpation) uses FIA data and expert opinion to group tree species under threat by non-native pests into vulnerability classes and specify appropriate management and conservation strategies. The scientists prioritized 419 tree species native to the North American continent. The analysis identified 15 taxonomic groups requiring the most immediate conservation intervention because of the tree species’ exposure to an extrinsic threat, their sensitivity to the threat, and their ability to adapt to it. Each of these 15 most vulnerable species, and several additional species, should be the focus of both a comprehensive gene conservation program and a genetic resistance screening and development effort.
While the Fraser fir ranks high under the criteria applied by the CAPTURE project, this project apparently doesn’t rank the Western firs.
Sources
Alsop, F. J., III, and T. F. Laughlin. 1991. Changes in the spruce-fir avifauna of Mt. Guyot, Tennessee, 1967-1985. J. Tenn. Acad. Sci. 66: 207-209.
American Phytopathological Society. Science Daily. December 9, 2019 https://www.sciencedaily.com/releases/2019/12/191209161314.htm?utm_source=feedburner&utm_medium=email&utm_campaign=Feed%3A+sciencedaily%2Fplants_animals%2Finvasive_species+%28Invasive+Species+News+–+ScienceDaily%29
Annand, P.N. 1928. A Contribution toward a monograph of the Adelginae (Phylloxeridae) of North America. Stanford Univ. Press, Palo Alto, Calif. 146 pages.
Balch, R.E., Clark J. and Bonga J.M. 1964. Hormonal action in production of tumors and compression wood by an aphid. Nature 202: 721–722.
Buhl, C. 2017. Oregon Department of Forestry, Forest Health Fact Sheet, June 2017.
Hay, R.L. 1978. Fraser fir in the Great Smoky Mountains National Park: Its demise by the balsam woolly aphid (Adelges piceae Artz.). Department of Forestry, Wildlife, and Fisheries, The University of Tennessee, Knoxville. 125 pages.
Hessl, A., A.M. Liebhold, and M.L. Leef. 2022. Dendrochronological Reconstruction of the Historical Invasion of Balsam Woolly Adelgid, Adelges piceae, Feeding on Canaan Fir, Abies balsamea subsp. phanerolepis in the Central Appalachian Mountains. Castanea, Vol. 87(1) 2022
Humble, L. M. 1994. Recovery of additional exotic predators of balsam woolly adelgid, Adelges piceae (Ratzeburg) (Homoptera:Adelgidae), in British Columbia. Can. Entomol. 126: 1101-1103.
Humble, L.M. Canadian Forest Service, pers. comm. June 2010
Johnson, N.E. and Wright, K.H. 1957. The balsam woolly aphid problem in Oregon and Washington. U. S. Forest Service Research Paper PNW 18: 1-34.
Johnson, K.D. 1980. Fraser fir and woolly balsam adelgid: a summary of information. Southern Appalachian Research/Resource Management Cooperative, Western Carolina University, Cullowhee, North Carolina. 62 pages.
Keen, F.P. 1938. Insect Enemies of Western Forests. United States Department of Agriculture, Miscellaneous Publication No. 273. U.S. Department of Agriculture, Bureau of Entomology and Plant Quarantine, Washington, D.C. 280 pp.
Kohler, G. Forest Entomologist, Washington Department of Natural Resources. 2016. Balsam Woolly Adelgid . Adelges piceae. https://westernforestry.org/wp-content/uploads/2016/06/Glenn-Kohler.pdf
Kotinsky, J. 1916. The European fir trunk louse, Chermes (Dreyjusia) piceae (Ratz.). Ent. Proc. Soc. Washington 18: 14-16.
Livingston, R.L., Dewey, J.E., Beckman, D.P. and Stipe, L.E. 2000. Distribution of the balsam woolly adelgid in Idaho. Western Journal of Applied Forestry 15: 227–231.
Lowrey, L. L. 2015a. Evaluation of the extent of the non-native insect, balsam woolly adelgid, south of the Salmon River in Idaho from 2006-2014 by ground delimitation and aerial detection surveys. Trip Report BFO-PR-2015-01.
U.S. Department of Agriculture, Forest Service, Intermountain Region, Forest Health Protection, Boise, ID. 9 p. (Accessed February 3, 2018).
Mitchell, R. G. and P. E. Buffam. 2001. Patterns of long-term balsam woolly adelgid infestations and effects in Oregon and Washington. West. J. APpl. For. 16: 121-126.
Montgomery, M.E. & Havill, N.P. 2014. Balsam woolly adelgid, chapter II. In The Use of Classical Biological Control to Preserve Forests in North America. FHTET-2013-2. (ed. by Van Driesche, R. & Reardon, R.). USDA Forest Service, Forest Health Technology Enterprise Team, Morgantown, West Virginia, pp. 9–19.
Pederson, L., L. Moffitt, J. Fidgen, D. Beckman, B. Burkhead, & N. Kittelson. No date. Balsam Woolly Adelgid Delimt in Idaho. Poster
Potter K.M. and B.L. Conkling. 2019. Forest Health Monitoring: National Status, Trends, and Analysis 2018. Forest Service Research & Development Southern Research Station. General Technical Report SRS-239. June 2019
Potter, K.M., Escanferla, M.E., Jetton, R.M., Man, G., Crane, B.S. 2019. Prioritizing the conservation needs of United States tree species: Evaluating vulnerability to forest insect and disease threats. Global Ecology and Conservation 18: e00622.
Rabenold, K. N., P. T. Fauth, B. W. Goodner, J. A. Sadowski, and P. G. Parker. 1998. Response of avian communities to disturbance by an exotic insect in spruce-fir forests of the southern Appalachians. Conservat. Biol. 12: 177-189.
Ragenovich, I.R. and R.G. Mitchell. 2006. Forest Insect and Disease Leaflet (FIDL) #118. https://www.na.fs.fed.us/pubs/fidls/bwa.pdf
Schooley, H.O., Harris, J.W.E., and Pendrel, B. 1984. Adelges piceae (Ratz.), Balsam Woolly Adelgid (Homoptera: Adelgidae). In J.S. Kelleher and M.A. Hulme (eds.). Biological Control Programmes against Insects and Weeds in Canada 1969-1980, Commonwealth Agricultural Bureaux, England, 1984.
United States Department of Agriculture Forest Service Forest Insect and Disease Leaflets – Balsam Woolly Adelgid Updated 2017; https://apps.fs.usda.gov/r6_decaid/views/balsam_woolly_adelgid.html
United States Department of Interior National Park Service. Date? Effects of Balsam Woolly Adelgid on True Firs in a Changing Climate; Mount Rainier National Park, North Cascades National Park
Zilahi-Balogh, G., Humble, L., Foottit, R., Burleigh, J. and Stock, A. 2016. History of the balsam woolly adelgid, Adelges piceae (Ratzeburg), in British Columbia with notes on a recent range expansion. Journal of the Entomological Society of British Columbia 113: 21-38.



